The contribution of host cell-directed vs. parasite-directed immunity to the disease and dynamics of malaria infections
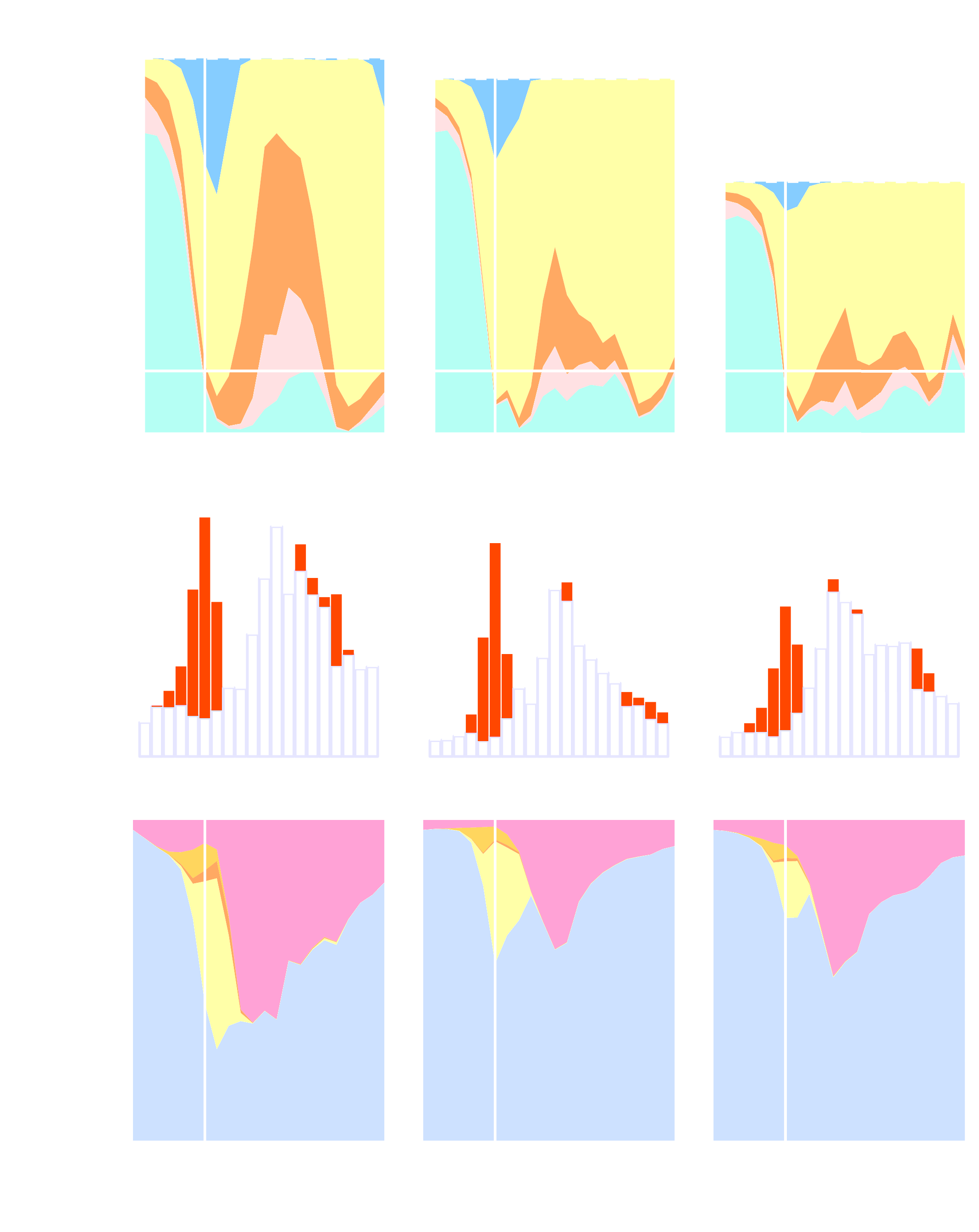
What makes an infected host sick–the pathogen or the host response to it? How should pathogens defend themselves? Answering these questions requires an understanding of how, and how much, the immune response changes populations of host and pathogen cells. We break down the immune response into components with distinct effects on cell birth and death, and quantify the impact of each on disease and pathogens. We find that hosts control infections not only by killing pathogens, but by starving parasites and shortening the lifespans of cells on which they depend. This work reveals that some immune responses–often seen as harmful to the host–may in fact be helpful and suggests simple rules that govern the immune response’s deployment.Hosts defend themselves against pathogens by mounting an immune response. Fully understanding the immune response as a driver of host disease and pathogen evolution requires a quantitative account of its impact on parasite population dynamics. Here, we use a data-driven modeling approach to quantify the birth and death processes underlying the dynamics of infections of the rodent malaria parasite, Plasmodium chabaudi, and the red blood cells (RBCs) it targets. We decompose the immune response into 3 components, each with a distinct effect on parasite and RBC vital rates, and quantify the relative contribution of each component to host disease and parasite density. Our analysis suggests that these components are deployed in a coordinated fashion to realize distinct resource-directed defense strategies that complement the killing of parasitized cells. Early in the infection, the host deploys a strategy reminiscent of siege and scorched-earth tactics, in which it both destroys RBCs and restricts their supply. Late in the infection, a “juvenilization” strategy, in which turnover of RBCs is accelerated, allows the host to recover from anemia while holding parasite proliferation at bay. By quantifying the impact of immunity on both parasite fitness and host disease, we reveal that phenomena often interpreted as immunopathology may in fact be beneficial to the host. Finally, we show that, across mice, the components of the host response are consistently related to each other, even when infections take qualitatively different trajectories. This suggests the existence of simple rules that govern the immune system’s deployment.
